Describe Alpha Helix Structure of Protein
When a number of successive peptide links have identical rotations the polypeptide chain takes up a particular secondary structure. Hierarchical Organization of Protein Structure Q31 - Describe the properties of an amphipathic alpha helix and identify numbered amino acids in a dodecamer polypeptide that contribute to the chemical properties of this secondary protein structure.

Alpha Helix An Overview Sciencedirect Topics
The α-helix is a regularly repeated polypeptide backbone structural motif that can be identified to varying degrees in the folded 3-D conformations of most proteins.
. And this structure appears as a rod that is wound around a central axis. When stability increases the. Of these the alpha helix is the commonest secondary structure of proteins.
The alpha helix is also called a classic PaulingCoreyBranson α-helix. The full 13 amino acid helix is shown in this view. An alpha helix sometimes called a Pauling-Corey-Branson alpha helix is a coil of amino acid chain.
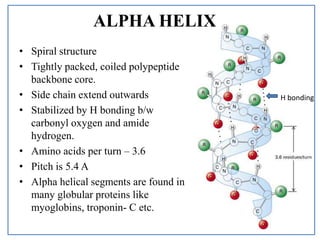
The alpha helix is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone NH group hydrogen bonds to the backbone CO group of the amino acid located four residues earlier along the protein sequence. Main Difference Alpha Helix and Beta Pleated Sheet. Alpha helix is a right handed-coiled or spiral conformation of polypeptide chains.
This is a secondary structure protein 3 dimensional right-handed spiral in shape or design which is stabilized by hydrogen bonds joining the NH group of an amino acid to a CO group of an amino acid in the next spiral. Break students into lab groups. The kinemage linked above shows an individual alpha helix viewed from the N-terminal end to resemble the helical wheel see figure below.
Alpha helix beta pleated sheet and different types of tight turns and explains most commonly found tight turn in proteins ie. Protein structure guided inquiry exercise Procedure. It almost always coils.
When these subunits come together they give the protein its quaternary structure. Secondary structure of protein is the conformation of protein in which polypeptide chain folds up by hydrogen bonding bet View the full answer. The screw sense of an alpha helix can be right-handed clockwise or left-handed counter-clockwise.
The turn of alpha helix we have been examining is a part of a longer alpha helix helix-4 located near the C-terminus of the ras protein. Students will be able to describe alpha-helices and beta-sheets structure and formation. Include which residues in the peptide backbone interact with each other and the forces that stabilise the secondary structure.
Briefs about the Ramachandran plot of proteins dihedral or torsion angles and explains why glycine and proline act as alpha helix breakers. In alpha helix every backbone N-H group donates a hydrogen bond to the backbone CO group which is placed in four residues prior. The 3-D image on the right highlights with.
Gives classification of secondary structure. Weve already encountered one example of a protein with quaternary structure. Complete the following paragraph to describe the structure of hemoglobin protein four At its simplest or sequence level hemoglobin is made up of a linear linked together by peptide bonds one samma pleated secondary The most common protein structures are the alpha helix and the sheet which are a result of each globin molecule.
Other helical structures include the 3_10 helix which is stabilized by hydrogen bonds of the type i i3 and the π-helix which is stabilized by hydrogen bonds of the type i i5. Again the peptide backbone is emphasized by showing the standard cartoon representations of secondary structure in which we trace only the peptide backbone as a helical cartoon without. However left-handed helices could also be present.
32 Secondary structure continued We can describe the arrangement of atoms around the peptide link the conformation by giving the degree and direction in which the Ca-CO and N-Ca bonds are rotated. The name 3613-helix is also used for this. Review basic functions of proteins and the structure of amino acids.
These structures are. Myoglobin Mb and its evolutionary cousins the α- and β-polypeptide chains of hemoglobin Hb exhibit unusually high percentages of α-helical structure more than 70. The α-helix is not the only helical structure in proteins.
Proteins have four structural levels of organization. Each Hydrogen bond that occurs in the alpha helix is parallel which can be seen in the picture below and at every turn of the alpha helix there are 36 residues present. 3 models of α-helix 2.
It is characterized by the spiral shape in which the amino acids are arranged which seem to be arranged around an imaginary longitudinal axis with the R groups. The alpha helix it is the simplest secondary structure that a protein can adopt in space according to the rigidity and freedom of rotation of the bonds between its amino acid residues. The folding or coiling of the primary structure into either an Alpha helix or beta pleated sheet.
The 3_10 helix has a smaller radius compared to the α-helix while the π-helix has a larger radius. Alpha helices α-helices are characterized by tight right-handed coils. In a tertiary structure the amino acids in the linear system is arranged spatially and the residues that are located adjacent to the sequence is also of spatial arrangement.
The Alpha Helix Structure. The most common shape found at the secondary level of protein structure is the alpha-helix. Attendance and administrative stuff.
Furthermore the alpha helix is a right-handed helix. Since an α helix has a repeat length of 36 amino acid residues per turn Figure 85 certain positions of a helixturnhelix domain will face toward the body of the protein a hydrophobic environment whereas certain residues will face the solvent or the DNA a hydrophilic environment. A similarity in the types of amino acids at these positions is observed within the.
Where are amphipathic alpha helices found in a globular cytosolic protein. Secondary protein structure The alpha- helix Below is an alpha helix add details to the diagram to describe its structure. Each turn of the spiral increases by 54 Angstrom 054 nm contains 37 amino acids on average side-groups protrude from the outside of the molecule are.
The O and N atoms of the helix main chain are shown as red and blue balls respectively. Alpha helix and beta plates are two different secondary structures of protein. Despite the fact that based on the Ramachandran plot both right-handed and left-handed alpha helices are among the permitted conformations the right-handed alpha helix is energetically more favorable because of fewer steric clashes between the side chains and the.
As mentioned earlier hemoglobin carries oxygen in the blood and is made up of four subunits two each of the α. An alpha helix is an element of secondary structure in which the amino acid chain is arranged in a spiral. What is Alpha Helix.

Representative Geometry Of An Alpha Helix Structure A The Coiled Download Scientific Diagram

No comments for "Describe Alpha Helix Structure of Protein"
Post a Comment